How Can We Cut and Repair Defective Genes?
How Can We Cut and Repair the Defective Genes?
by Marwa Zafarullah
Advanced genome editing tools improve our ability to cut and repair the defective genes responsible for various diseases in human beings, along with the generation of animal models and agricultural applications. Now the questions are, how does genome editing work? What are the platforms and applications? What are the challenges faced?
When you need to repair something, you go to the hardware store and buy the appropriate tool. Similarly, when scientists need to repair a defective gene, they go to their laboratories and create the appropriate tools. For decades scientists have been developing technologies that give them the ability to change an organism's defective genetic material responsible for various disorders.
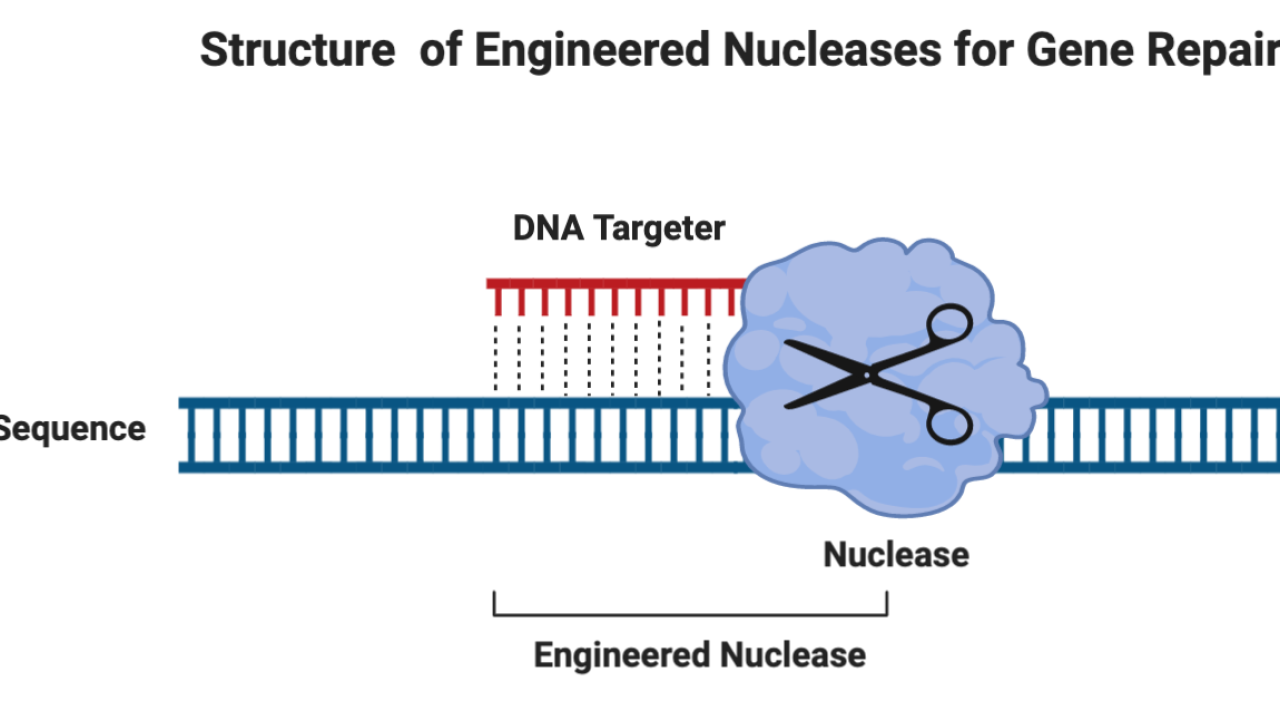
How does genome editing work? The approach involves cutting the defective sequences of hereditary material called deoxyribonucleic acid, or DNA, at specific locations within the gene and either delivering corrected sequences to the sliced sites or letting cells naturally repair the cut, which removes the cause of the disease by restoring the target gene to its normalized function. Like any job, the gene repair requires the right instruments. In this case, we need the scissors, called engineered nucleases; they are the enzymes (proteins that significantly speed up the chemical reactions taking place within cells). All of the engineered nucleases consist of two parts (Figure 1). The first part is the nuclease that cuts the specific DNA sequence. The second part is the DNA-targeting nucleic acid that guides the nuclease to a specific DNA sequence. Together, these two parts cut out the faulty section of the gene and help normal repairing afterward.

Genome Editing Platforms
In the past years, there has been a vast emergence of highly versatile genome-editing platforms facilitating the gene modifications in a variety of cell types and living organisms. The most commonly used technologies these days are zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas9). All of these technologies have a nuclease to cut DNA and a DNA targeter to identify the specific sequence to be cut; the only difference is in their way of recognizing that particular sequence (Figure 2).
- Zinc-finger nucleases (ZFNs): This technology contains the FokI nuclease to cut the DNA sequence. While the Zinc-finger proteins help in identifying the specific sequence of the DNA. In action, two FokI nuclease, one for each strand of the DNA, come together to cut both sides, which later repaired with the help of cell repairing machinery. Although this technology was tested in various organisms, the difficulty of engineering and delivering ZFNs system presents a significant limitation.
- Transcription activator-like effector nucleases (TALENs): Like ZFNs, TALENs also contain the FokI nuclease to cut the DNA sequence. While it recognizes the specific sequence on the DNA with the help of the transcription activator-like effector (TALE) conserved protein. In action, two FokI nuclease, one for each strand of the DNA, come together to cut both sides, so two TALENS have been designed to modify one DNA molecule. This technology provides better specificity and efficiency than ZFNs while requiring more complex system engineering due to the large size of functional units.
- Clustered regularly interspaced short palindromic repeats (CRISPR-Cas9): This is one of the most popular, widely used, and efficient systems of gene cut and repair. This platform used CRISPR-associated protein 9 as nuclease to cut the sequence of the DNA. While the specific single-stranded guided ribonucleic acid (sgRNA) directed the Cas9 nuclease to the target location. Due to the universal identity of nuclease protein Cas9 in all living organisms, genome editing becomes easier and more scalable than other technologies.

Applications of Genome Editing Technologies
Advanced genome editing technologies, especially CRISPR-Cas9, have opened new avenues of biotech research applications, ranging from the development of better cellular and/or animal models, to curing genetic diseases and improving agricultural production (Figure 3).
- Generation of cellular and animal models: Genome editing technologies providing a versatile approach to creating cellular and animal models, including worm, rat, rabbit, pig, and monkey, to better understand disease development mechanisms and potential treatments. In addition, with faster generation of these useful models, a dramatic decrease in research time and resource consumption has been observed.
- Curing of genetic diseases: Out of 25,000 identified genes in the human genome, changes in over 3,000 have been linked to different genetic diseases. New genome editing technologies are now being used to understand how these gene changes are related to human disorders. CRISPR/Cas9 research in mouse models can currently correct changes in faulty genes responsible for Hepatitis B, hemophilia, severe combined immunodeficiency, cataracts, cystic fibrosis, hereditary tyrosinemia, and inherited Duchenne muscular dystrophy. In addition, clinical trials are underway to modify immune-system cells' genes to treat HIV via ZFNs.
- Agricultural Improvements: With projected increases in the global population, food scarcity will likely be a significant future challenge. Genome editing technologies have opened the door to creating a range of crop varieties, including maize, rice, and wheat, with desirable traits, such as drought tolerance and pest and disease resistance, which not only help in decreasing pesticide, fertilizer and water usage, but improve food quality and safety. These tools have also been used to enhance the quality of livestock animals' lives, such as the hornless dairy cattle that have been produced to avoid the need for painful de-horning. The spread of contagious viruses like African swine fever and bird flu may also be controlled by the editing the susceptibility genes in domestic lines of pigs and chickens, respectively.

Technical and Ethical Challenges
Although the described genome editing technologies have many useful applications and the therapeutic potential to eliminate defective genes for the effective treatment of various disorders, there are a few technical and ethical challenges that need to be addressed. One of the biggest technological challenges is preventing "off target" or non-specific cutting in the genome. However, with the advancement of the technology, off-targeting is being minimized. Still, there is a lot of improvement required for use in new precision medicine approaches. In addition, there has been on-going discussion around the world regarding the use of technologies to edit genes that will be passed on to the next generation. People worldwide do not share a standard view on the act of choice where creating and altering life is concerned. Broadly, there are currently two schools of thought, one that opposes genome editing with some religious logic, and another that supports the idea of genome editing for improving the human condition through the elimination of harmful characteristics. At the same time, there are solid ethical regulations in practices in some parts of the world, while less in others. Recently the Chinese biophysicist He Jiankui announced that he had created the world’s first gene-edited babies with CRISPR technology. That was a big shock to the world. Though this emerging technology looks very promising in plants, animals, and human cell models, there is still a lot of needed optimization to eliminate off-target effects. The current scientific consensus is that this technology is not ready to be used on humans. For illegal medical practices, He Jiankui has been sent to prison by the Chinese government for three years (Press Release).
Conclusion
On-going refinement of genome editing tools, including ZFNs, TALENs, and CRISPR-Cas, are improving our ability to cut and repair defective genes in plants, animals, and ourselves. Genome editing may eventually lead to a revolution in healthcare by paving the path to personalized medicine. But, there is still work to be done. We will need for economists and regulators to ensure safe, effective, and affordable outcomes, as the potential impacts on patients and society will be great.
Images in this post have been created with BioRender.com.
