The Future of Medicine: Gene Therapy and Its Applications
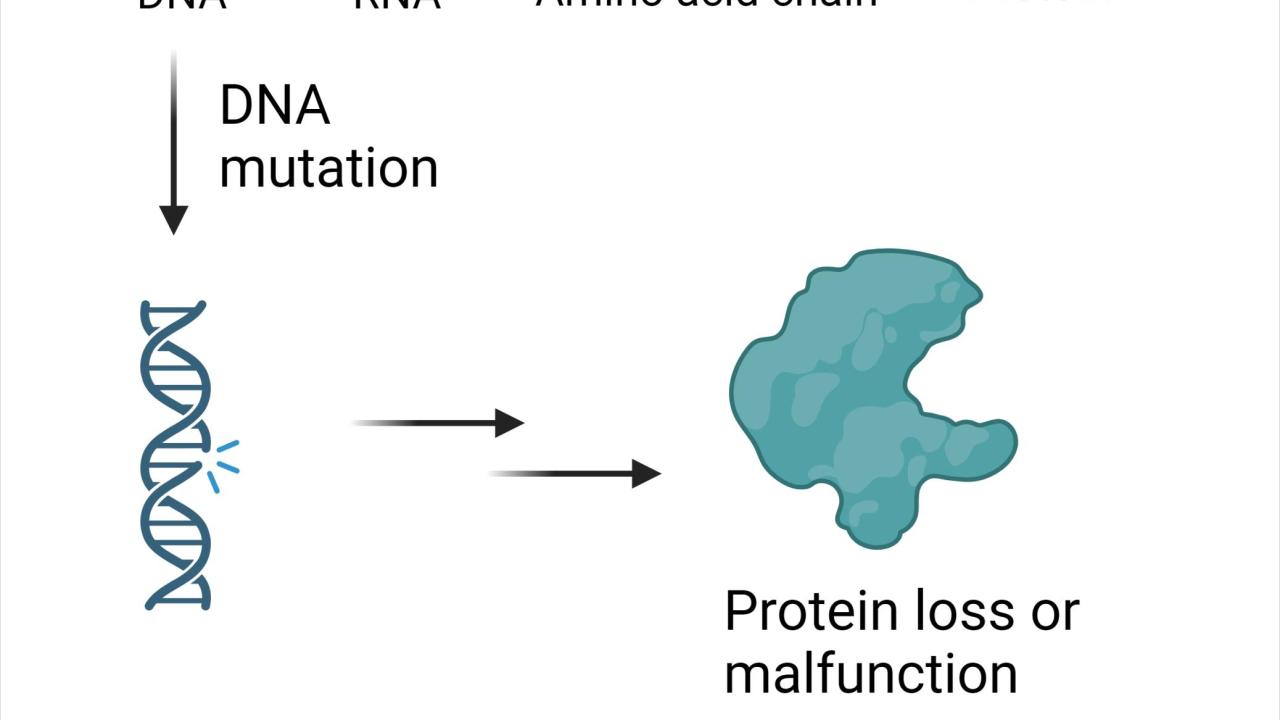
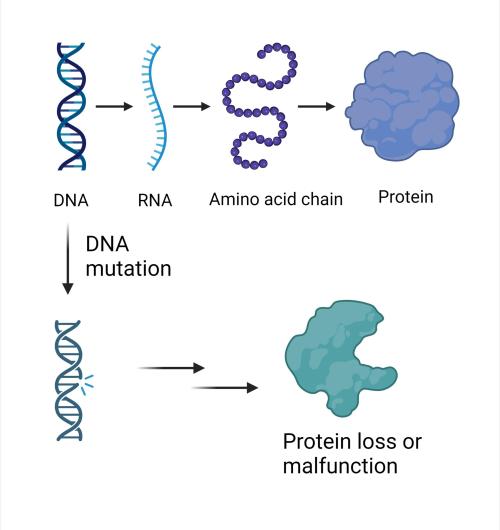
Tall or short? Blue irises or brown? Human genetic material, or deoxyribonucleic acid (DNA), include genes that provide cellular machinery with instructions to produce functional proteins. These proteins ultimately determine many traits and support our daily activities. However, due to cell aging or environmental exposure to chemicals or radiation, mutations can happen randomly within trait-encoding genes, leading to problematic gene expression and protein activity (Figure 1). If mutations occur within germ line cells, those that give rise to sperm and egg gametes, non-functional genes can be passed to offspring and lead to heritable genetic diseases.
Gene therapy is a method of treatment that fixes the root problem of heritable genetic diseases, instead of merely alleviating the symptoms. One gene therapy approach involves delivering whole therapeutic gene sequences into cells which can be transcribed and translated to produce functional protein - this is known as gene addition. Genes that are incorrectly overexpressing too much protein may also be "turned down" using a similar approach - this is known as gene silencing. Another gene therapy approach is gene editing, such as the use of the novel technology CRISPR-Cas9, to introduces molecular tools to "fix" the mutated gene sequence found in the cell's DNA.
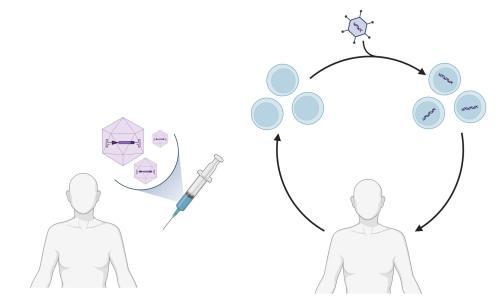
There are two general strategies for gene therapies: in vivo and ex vivo (Figure 2). In in vivo gene therapy, the therapeutic genetic material is delivered directly into the patient's cells or tissues, without ex vivo manipulation of cells. This can be achieved through various methods such as viral vectors (Figure 3), non-viral vectors, or physical techniques like electroporation. Although long-term and stable expression of the introduced genetic material is possible, the efficiency of gene delivery requires further improvement. Ex vivo gene therapy requires that cells are first collected from the patient, genetically modified, and then returned to the patient. Although the ex vivo gene therapy allows precise genetic modification of cells in a controlled laboratory setting, the process can be time-consuming and costly due to the requirement of specialized laboratory facilities and expertise. The extraction and manipulation of cells outside the body also carries the risk of contamination or damage to the cells.
In principle, gene therapy corrects errors in gene expression and heritable genetic diseases can be treated or cured - progress has been made in gene therapy approaches for many diseases. However, fewer than 40 gene therapy medicines have been approved for sale in the past 10 years. One reason for the small number of available gene therapies is that scientists still know very little about the nature of many heritable diseases and the corresponding genetic information and mutations. Without knowledge of the underlying mutations and disease mechanisms, it is not possible to design gene therapies to fix these errors.
In addition, gene targeting and delivery efficiency into target tissues and cells is still low. Some gene editing tools still have a high off-target rate, meaning that non-target genes might be mutated in the process of trying to fix a target gene. Despite these challenges, gene therapy is still gradually moving steadily and cautiously from theoretical research into clinical practice with the progress of biotechnology.
Gene Therapy Regulatory Milestones
- On December 18th, 2017, the U.S. Food and Drug Administration (FDA) approved Luxturna to cure an inherited form of vision loss for both children and adults. The normal gene RPE65 was delivered via an adeno-associated virus (AAV)-based vector. The healthy gene was directly delivered to the retinal cells and expressed the normal protein to help restore vision.
- On May 24th, 2019, FDA approved Zolgensma for children younger than 2 years old with spinal muscular atrophy (SMA), caused by mutation on the survival motor neuron (SMN) gene. The abnormal gene expresses little SMN protein which is critical to the maintenance and function of specific nerve cells: motor neuron cells. Zolgensma delivers the functional SMN gene into motor neuron cells via an adeno-associated virus (AAV)-based vector. This therapy increases the expression of SMN protein in infant patients and therefore improves their muscle movement and functions.
- On November 17th, 2023, Casgevy, a gene-editing therapy based on CRISPR-Cas9 technology aiming to cure sickle cell disease and transfusion-dependent β-thalassemia, was approved for listing in the UK, which is also the world's first approved CRISPR gene-editing therapy.
- On December 8th, 2023, Casgevy and Lyfgenia were both approved by the FDA as cell-based gene therapy treatments for sickle cell disease (SCD) for patients 12 years and older. In both treatment methods, blood stem cells are collected, and the cell and gene modifications were applied ex vivo. Casgevy was also the first approved by the FDA using CRISPR-Cas9 technology. The genes in the blood stem cells are modified and transplanted back into the patient. Cells with modified genes in bone marrow will multiply and increase fetal hemoglobin (HbF), a type of hemoglobin that facilitates oxygen delivery, which can prevent sickling of blood cells. While Lyfgenia requires a lentiviral vector to deliver genetically modified blood stem cells back to patients. The modified genes can increase the production of HbAT87Q, a gene-therapy-derived hemoglobin that functions similarly to a protein expressed in healthy adults. Red blood cells containing HbAT87Q have a lower risk of sickling.

Outlook for Gene Therapy Approaches
Researchers and scientists are still investigating the complex relationship between diseases or disorders and the genes behind them. Information on human functional genomics and genotype-phenotype relationships is always emerging. For example, it has been reported that a mutation or disruption of the SH3 and ankyrin repeat domains 3 (SHANK3) gene can cause autism-like symptoms and potentially increase susceptibility to autism spectrum disorder. In January 2024, Jaguar Gene Therapy was permitted by the FDA to start human trials with JAG201, which delivers a functional copy of the SHANK3 gene via an adeno-associated virus vector. Despite the limited accessibility due to delivery methods, high costs, and ethical concerns, gene therapies can be tailored to individual patients and address the root causes of diseases at the molecular level, introducing a new era of precision medicine. As technology advances and our understanding of genetics deepens, we can expect increasingly sophisticated and effective gene therapy approaches. Together, let us unveil the future of medicine—one gene at a time.
All graphics in this blog were generated with BioRender.com.
References
Luo, R., Le, H., Wu, Q., Gong, C. Nanoplatform‐based in vivo gene delivery systems for cancer therapy. Small, 2024, e2312153.
Bulaklak, K., Gersbach, C.A. The once and future gene therapy. Nat. Commun., 2020, 11, 5820.
FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss | FDA
FDA Approves First Gene Therapies to Treat Patients with Sickle Cell Disease | FDA
Zhou Y, Sharma J, Ke Q, et. al., Atypical behaviour and connectivity in SHANK3-mutant macaques. Nature, 2019, 570(7761): 326-331.
