
Current Treatments and Trials Against COVID-19
Current Treatments and Trials Against COVID-19
by Alan Nguyen
At the time that I write this blog, it was only a few months ago that things were still “normal”. By “normal”, I mean that we were still able to attend social outings, such as going to the movie theaters, eating out at restaurants and bars, or simply enjoying the company of our friends at their places. Then, everything was brought to a screeching halt when the covid-19 pandemic was established. All restaurants and bars are no longer serving meals for dine-in customers – everything is either take-out or delivery. Schools are closed for the remainder of the academic year, and all learning is done online. All professional sport leagues, concerts, and religious in-person gatherings are indefinitely suspended. In addition, everyone is placed under a stay-at-home mandate and directed to practice “social distancing” in which everybody must remain a distance of at least six feet from each other.
For the first few weeks, people started to panic and began hoarding essential items such as hand sanitizer, toilet paper, paper towels, hair products, flour, fresh produce, and eggs. The sight of depleted store shelves became a common sight and managing to get your hands on any of these essential products was considered an accomplishment. I remember not seeing any stock of toilet paper or paper towels at the local grocery stores for about three weeks. Don’t worry though, I did end up getting my hands on a pack of 9 rolls from Walgreens and I am currently stocked on groceries and food at my home.
Recently, Governor Gavin Newsom made an announcement detailing his plans for reopening California’s economy. One of the many parameters he listed out for reopening of the state include engagement of academic researchers at various universities and institutions (i.e. University of California, Stanford, USC, Scripps, etc.). He also mentioned that the normalcy that we were used to before the covid-19 pandemic may not return until there is an establishment of herd immunity and/or a development of a vaccine. Fortunately, there have been a number of clinical trials and research investigations of vaccine development and treatments against covid-19. Here, I will discuss the different types of available treatments that are being tested for covid-19, the efforts being made to develop a vaccine, and ways scientists and doctors are using to detect the virus that causes covid-19.
The reason why we are implementing the shelter-in-place orders is that the virus that causes covid-19, or SARS-CoV2, is highly infectious and the general population has yet to establish immunity. This makes it extremely easy for the virus to spread and infect a plethora of people in communities. There is a reason why SARS-CoV2 is called a novel coronavirus – it’s because it’s never been encountered before and there is a lack of herd immunity against the virus. Herd immunity is the concept in which the majority of the population is immunized against the virus, which provides a secondary form of protection for those who have yet to develop immunity against the infectious agent (figure 1). There are two main ways that herd immunity is established. The first method is for a majority of the population to become infected and recover from the virus. As a person becomes infected by a virus and gets sick, their immune system mounts a response to counter the infection. If the infection gets cleared, what usually happens is that the host will have generated antibodies against the virus. These antibodies tend to be long-lasting and may exist for the remainder of the person’s life. When this happens, the person can no longer be infected by the virus again and is now considered immune against the virus. In other words, the person’s immune system has generated a “memory” response against the virus. However, in the case of covid-19, we are unsure about the immunity patients who have recovered from the infection, and further testing (i.e. analysis of antibodies) is needed before we can make any conclusions about establishing immunity to the virus after being infected.
Obviously, it is best to not establish herd immunity to covid-19 by getting everyone infected, so that leaves the other major method – becoming immunized via a vaccine. To help accelerate herd immunity, researchers are attempting to develop a vaccine against this novel coronavirus. Some of these vaccines are currently undergoing phase I clinical trials and target the spike (S) protein that is expressed and used by the coronavirus to infect human cells [1]. The different characteristics of the vaccines being developed include mRNA vaccines, adenoviral, DNA plasmid, and lentiviral vectors that contain either a nucleic acid or amino acid sequence (protein) that is expressed only by SARS-CoV2. These vaccine characteristics allow scientists a multitude of methods to deliver the target protein in the hopes of generating an immune response in the host. Other methods of vaccine development against covid-19 include modification of live immune cells that can express parts of viral proteins [1]. The modified immune cells can then be administered into the host. Through this method, the host’s lymphocytes (T cells) can recognize the viral proteins expressed by the injected immune cells and initiate an immune response against the target, thus conferring immunity against the virus. Currently, there are no approved vaccines against covid-19.
The first clinical trial for a vaccine against covid-19 was launched at Kaiser Permanente Washington Health Research Institute in Seattle in March 2020, led by Dr. Lisa A. Jackson. The vaccine being tested was developed by Moderna Inc. and was synthesized by embedding mRNA that encodes for the coronavirus S protein and embeds it in a lipid nanoparticle. Remarkably, this clinical trial was launched within two weeks as the coronavirus was beginning its spread throughout the United States (Reference: https://www.niaid.nih.gov/news-events/nih-clinical-trial-investigational-vaccine-covid-19-begins). In this trial, 45 patients are being tested over 6 weeks and are receiving two doses of the vaccine 28 days apart. All patients will return to the clinic for follow-up visits between vaccinations and will continue to do so for a year after their second dose. Ultimately, the readout from these patients will be blood tests which will determine whether they have mounted an immune response against SARS-CoV2 by looking for the presence of virus-specific antibodies.
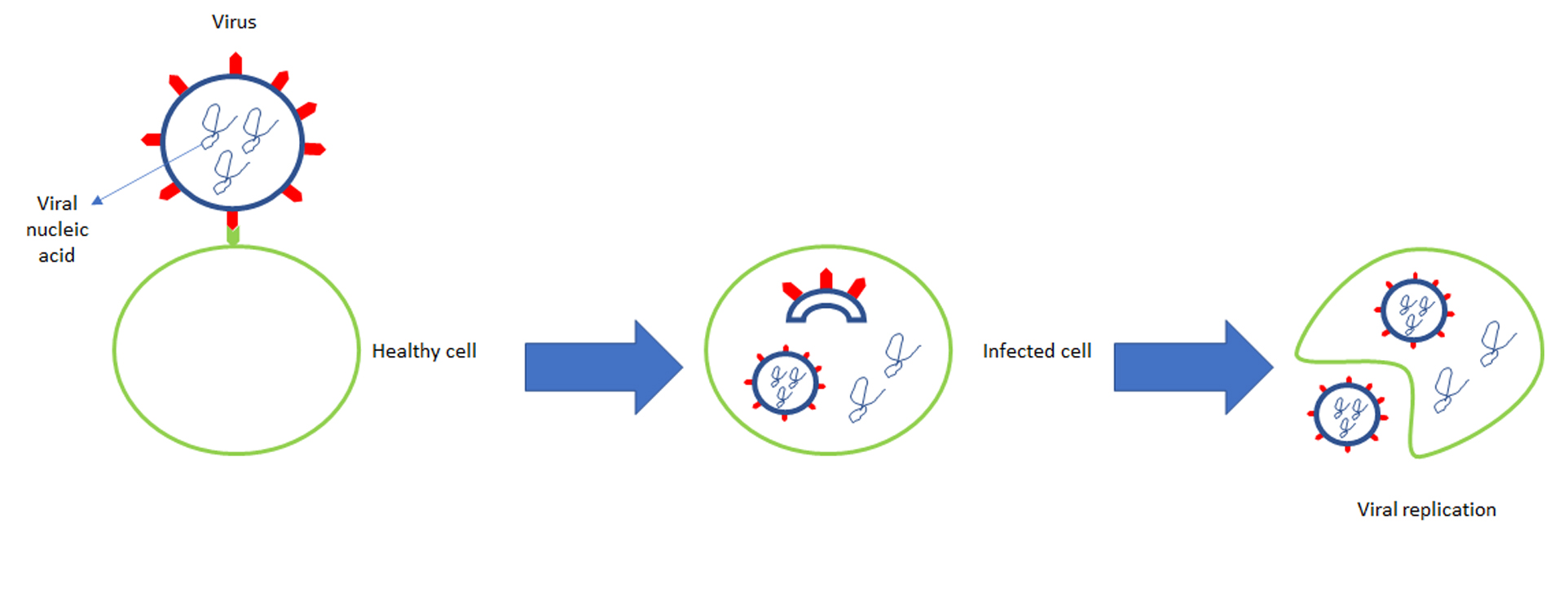
While scientists are hard at work developing a vaccine to help establish herd immunity, others are working on treatments to help those who are currently infected with the virus. One of the major drugs being tested for use against covid-19 right now is Remdesivir. This drug is produced by Gilead, and works by inhibiting the machinery that allows the SARS-CoV2 virus to replicate itself. Remdesivir has been shown to effectively block RNA polymerases [2-4], which is required for coronaviruses to replicate in an infected host [5]. A recent trial revealed that 68% of covid-19 patients improved in oxygen support after Remdesivir treatment [6]. The same study also showed that 57% of patients who were receiving invasive mechanical intubation were extubated after Remdesivir treatment. Most recently, Dr. Anthony Fauci has commented on the efficacy of Remdesivir, reporting that the median time for recovery was about 11 days, which was a shorter timespan when compared to the placebo group (Reference: https://www.cnbc.com/2020/04/29/dr-anthony-fauci-says-data-from-remdesivir-coronavirus-drug-trial-shows-quite-good-news.html). Dr. Fauci also mentioned that the mortality rate of patients treated with Remdesivir was 8% vs. 11.6% for the placebo-treated patients. With the recent findings from the Remdesivir trial, there is a high amount of potential for this drug to be put into wide use for covid-19 patients.
Another promising treatment that is being tested is plasma from patients who have been infected and recovered from covid-19. As mentioned earlier, patients who have recovered from covid-19 should have generated an antibody response against the virus. In theory, these antibodies should neutralize the virus and assist other covid-19 patients in their fight against the virus. A study in China showed that covid-19 patients had a reduction in the detectable levels of the SARS-CoV2 virus when they were transfused with the plasma of patients who have recovered from the infection. The patients who received the plasma also showed elevated titers of neutralizing antibody shortly after transfusion [7]. However, this study included a small number of patients and further testing is needed to yield more conclusive results.
Other drugs show potential for use against coronaviruses. Japanese scientists have shown that Favipiravir, another drug that can inhibit RNA polymerases, was shown to be effective against multiple types of viruses, such as arenaviruses, bunyaviruses, flaviviruses, and picornaviruses [8]. However, it has yet to be tested on coronaviruses, but its use against covid-19 does seem promising.
Here at UC Davis, two trials for treatments against covid-19 have been launched. The UC Davis Medical Center is one of 75 sites worldwide testing Remdesivir for severe covid-19 infection. This study will involve 10 or more patients in Sacramento and is currently being led by Dr. Stuart Cohen, who is the chief of the Division of Infectious Diseases at UC Davis Health. Another drug being tested by UC Davis is Sarilumab, which is a drug that is currently used for treating rheumatoid arthritis. This drug is an antibody that blocks the activity of an inflammatory molecule called interleukin-6. While inflammation is often a positive sign that the immune system is working against an infectious agent, too much inflammation can often lead to complications, such as organ failure, or death for patients. Thus, blocking interleukin-6 in severe covid-19 patients may be beneficial.
Collectively, the current treatments and clinical trials for covid-19 represents progress in the fight against the pandemic. While we’ll have to adjust to the new “normal” here in the United States, there is hope that we will return to the normalcy we were so used to before the pandemic. It may not be too long from now that we can once again go back to restaurants and bars, sporting events, and our friends’ houses, but that won’t become a reality once we have created the herd immunity to covid-19. With the efforts of hard-working scientists, doctors, and nurses, we may soon find that bridge to herd immunity.
