Biomarker Development: A Tool to Prevent Neurodegenerative Disorders
Biomarker Development: A Tool to Prevent Neurodegenerative Disorders
By Marwa Zafarullah
Identification and validation of reliable biomarkers can improve diagnosis, monitor therapeutic response, and guide the development of targeted therapies for neurodegenerative disorders. Now the questions are, what are these biomarkers? How can we develop them? What are the challenges faced?
What are Biomarkers?
The National Institute of Health Biomarkers Definitions Working Group defined a biomarker as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.” In other words, “Biomarkers are a naturally occurring molecule, gene, or characteristic by which a particular pathological or physiological process, and disease can be identified.” According to the Food and Drug Administration (FDA), an ideal biomarker has the following features that make it suitable for testing a particular disease condition.
- Cost-efficient.
- Safe and easy to measure.
- Modifiable with treatments.
- Able to give accurate results.
- Rapid to enable faster diagnosis.
- Should express early in disease progression.
- Consistent between different ethnic groups and genders.
- Specific for a particular disease and able to differentiate between different physiological states.
Why need Biomarkers for Neurodegenerative Disorders?
Neurodegenerative diseases represent a big group of neurological disorders with varied clinical and pathological expressions affecting a particular subset of neurons in specific functional systems. Today, in a modern world, neurodegenerative disease represents one of the significant causes of death. Across all the medical disciplines, we have been presented with a disheartening and provocative issue. The number of age-dependent disorders is increasing immensely in the past couple of years due to an increase in the elderly population. According to the 2019 Alzheimer report out of the total U.S. population, about 5.8 million Americans of all ages or one in 10 people age 65 and older has Alzheimer’s dementia. If it left unchecked 30 years from now, more than 12 million Americans would suffer from neurodegenerative diseases. Unfortunately, despite new technologies, the production rate of new effective medications for neurodegenerative disorders has been dropping as FDA approved therapeutic drugs for most common neurodegenerative diseases like Alzheimer’s and Parkinson’s disease are based on three to four decades of research work.
Now, the most important question has become, “Where have we gone wrong?”. It is self-evident that the translation of basic science research to the real-world benefit of drug development is becoming less. As the development of novel targeted therapeutics faces many challenges, including the lack of biomarkers for early diagnosis and progression, limited historical data, complicated route to FDA approval, and difficulty of assessing effectiveness in clinical trials for which small patient populations limit enrollment. Another possible critical roadblock to finding cures for neurodegenerative disorders is the inadequate availability of high-quality, highly characterized human brain tissues for translational research.

In the past decade, it has been firmly accepted that most of the neurodegenerative disorders develop gradually with a long asymptomatic period, during which biomarkers might help in early diagnosis and for tracking an unseen disease progression. It brings a significant shift towards trying to prevent neurodegenerative disorders from happening, rather than curing after occurring. Thus, it is apparent that the identification of robust and validated biomarkers, which can improve diagnosis, monitor therapeutic response, and guide the development of safer and targeted therapies for various neurodegenerative diseases, is utterly required. Recently, the advancement in multi-omics approaches (e.g., genomics, transcriptomics, proteomics, and metabolomics) combined with bioinformatics and biostatistics accelerates the whole process of biomarker development [Fig. 1]. Besides, it is expected that this biomarker development will improve diagnostic accuracy and clinical trial efficiency.
Types of Biomarkers for Neurodegenerative Disorder
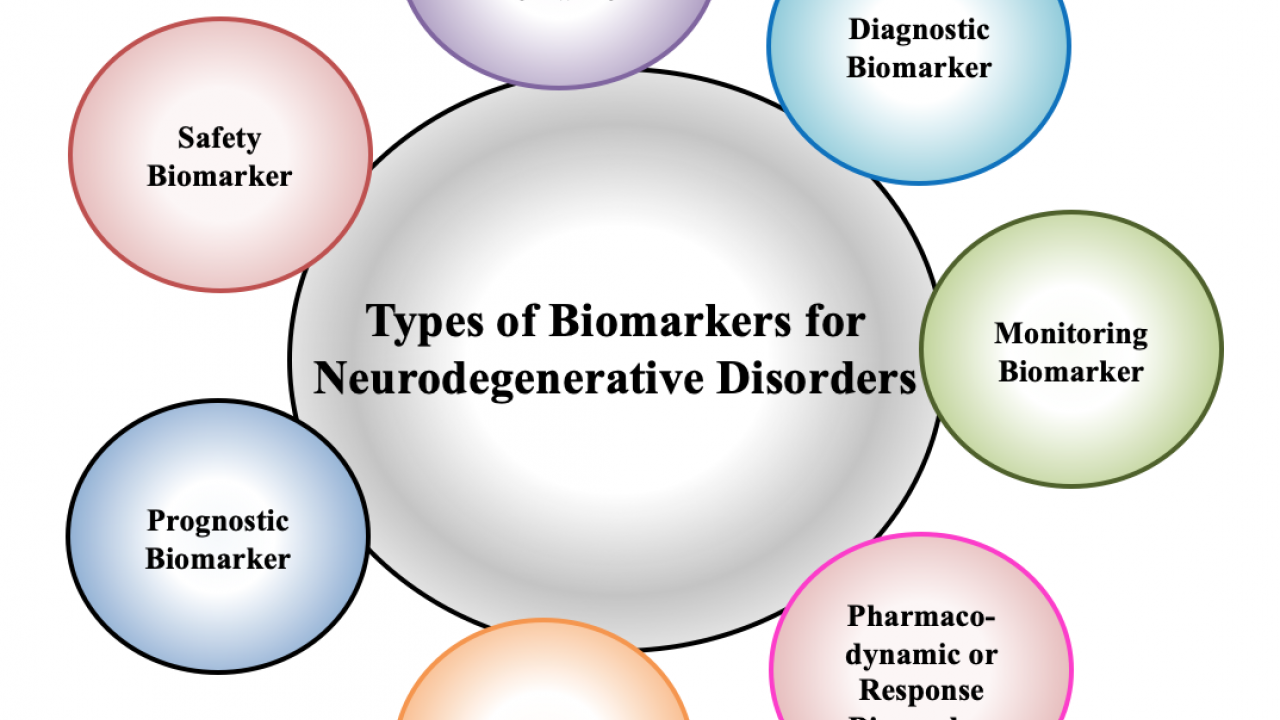
According to the FDA-NIH Biomarker Working Group, there are seven types of biomarkers for neurodegenerative disorders based on their specific use or the clinical application. It includes diagnostic, monitoring, pharmacodynamic, or response, predictive, prognostic, safety, and susceptibility/risk biomarkers, as shown in [Fig. 2].
- Diagnostic Biomarkers: These biomarkers are used as a diagnostic tool to detect or confirm the presence of neurodegenerative disorder, and its subtypes. These biomarkers would also contribute to improving personalized medicine by increasing the effectiveness of the therapeutic response.
- Monitoring Biomarkers: These biomarkers are used to monitor the specific neurodegenerative disease prognosis or a reaction to an intervention. They can be applied in clinical trials, for medical product development, and to evaluate the risk of disease development.
- Pharmacodynamic or Response Biomarkers: These biomarkers determine the progression of the treatment and can be used as a potential tool in clinical practice to guide patient management. They are also of particular interest in early therapeutic drug development, as help in establishing the proof if the drug inducing the pharmacodynamic changes in humans compared to its clinical benefit.
- Predictive Biomarkers: These biomarker's presence or modification allows us to predict which patients are more likely to experience the effect of being exposed to a medical product. These biomarkers are very frequently used in controlled clinical trials of the new therapies as if the biomarker predicts a favorable outcome; most probably, its presence indicates a massive effect of the new treatment as compared to the control.
- Prognostic Biomarkers: These biomarker's presence allows the identification of populations at higher risk by identifying the patients more likely to develop neurodegenerative disease progression. Besides, they can also provide useful information about treatment safety and guiding patient hospitalization.
- Safety Biomarkers: These biomarkers allow us to identify the possibility of developing toxicity signs or detect the presence of toxicity before and after the exposure to medical intervention. All safety biomarkers have a collective ability to detect or predict toxicity (by changing their level) before the onset of clinical signs and irreversible damage. Also, they are beneficial in identifying patients in which particular therapies should not be started due to significant safety risks.
- Susceptibility or Risk Biomarkers: These biomarkers are used to evaluate the risk of developing a specific neurodegenerative disease and help in establishing a preventive strategy in clinical practice.

Framework for Biomarker Development.
Biomarker development for neurodegenerative disorders typically occurs in three consecutive phases, as shown in [Fig. 3]. These three phases comprise discovery, validation, and qualification.
- Discovery: A process of biomarker discovery starts with the analysis of a vast number of analytes and tests in a small number of samples or individuals, leads to the study of a promising smaller number of analytes in many samples or individuals. The discovery process can be conducted using biofluids or tissue extracts to identify candidate biomarkers. For the discovery phase to be successful, the comprehensive phenotyping of samples source (individuals or human), bioinformatics, pilot validation, and high-power detection methods are required.
- Validation: Validation is needed to determine whether a biomarker can be considered fit for a specific purpose. There are two types of validation Analytical validation and Clinical Validation. In analytical validation, establish the context of use, acceptability of performance characteristics, and test the precision, sensitivity, accuracy, and dynamic range of identified biomarkers. While on the other hand, for clinical validation test, the discovered biomarkers in large clinical studies, evaluate overall utility for the clinical practice and drug development.
- Qualification: Biomarker qualification in samples during the discovery process provides confidence that the biomarkers might have specific clinical services. The FDA and European Medicines Agency (EMA) have formal approval processes to lead the qualified biomarkers into the drug discovery process.

Challenges of Biomarker Development
In past decades, a great effort has been made to identify novel biomarkers by using advanced omics technologies. However, there are various challenges to translate them into clinical settings. The significant issues that need to be addressed for successful biomarker discovery and validation programs include the criteria for subject selection, kind of sample, collection and handling procedures, accurate data acquisition, analysis, and documentation. The testing of clinical sensitivity and specificity of biomarkers safety, ease of measurement, reduced costs of the clinical tests, consistency across different age groups, genders/ethnic groups, and the behavior of biomarkers are of great importance. Finally, before and implementation to the clinical level, the issues related to proof-of-concept, incremental value, clinical utility, external validation, cost-effectiveness, and clinical outcome have to be addressed. Apart from the concerns discussed above, a significant hurdle in the road to success is the outrageous cost of biomarker validation and the time required for a biomarker to be approved after passing all the phases of clinical trials. Due to all such limitations, very few neurodegenerative biomarkers have been approved by the FDA to date.
Conclusion
The search for biomarkers of neurodegenerative disease has longed shadowed the evolution of therapeutics itself. Although the diagnosis and progression of these disorders have traditionally leveraged by clinical factors, the personalized application of selective treatments can only be guided by positive biomarkers development. In addition, the increasing multi-disciplinary collaboration within and among academia and government agencies is required to validate the identified biomarker across the clinical settings. Looking forward, biomarker development promises to remain a critical determinant as to whether personalized care can reach its full potential for patients suffering from various neurodegenerative disorders.
Note: Images in this post have been created with BioRender.com.
