The Golden Era of RNA-Based Therapeutics
The development of mRNA vaccines against COVID-19 has showcased the potential of RNA-based approaches, generating significant enthusiasm and investment in the field. RNA-based therapeutics offer new potential treatments for a range of diseases, taking advantage of the natural role of RNA in gene expression and allowing for targeted gene regulation and protein synthesis. In this blog, we will explore the types of RNA-based therapeutics, their advantages and limitations, and the current state of research in the field. But first, let’s define what an RNA molecule is.
RNA, or ribonucleic acid, is a molecule that is structurally similar to DNA, or deoxyribonucleic acid, but differs in a few key aspects. Firstly, RNA is typically single-stranded, while DNA is double-stranded. The single-stranded structure of RNA allows it to fold into different configurations and interact with other molecules in a more flexible manner. This is why RNA has more diverse cellular functions compared to DNA, whose primary role is to store and transmit genetic information. Additionally, RNA contains the sugar ribose, whereas DNA contains deoxyribose. This variation hugely affects stability and reactivity of these molecules, such that RNA is inherently less stable and prone to nuclease degradation. These are the major reasons for the lag in the development of RNA-based therapeutics compared to DNA-based therapeutics. However, researchers have developed several strategies to overcome these issues, including the use of chemical modifications to increase stability and efficacy of RNA molecules in cells.
There are several types of RNA-based therapeutic approaches, each with its unique mechanism of action and potential therapeutic applications. These include:
- Messenger RNA (mRNA) therapy. mRNAs are naturally occurring molecules involved in the transfer of genetic information from DNA to the cellular machinery responsible for protein synthesis or translation. In mRNA-based therapeutics, synthetic mRNA molecules are engineered and delivered to cells to produce specific proteins or trigger immune responses for therapeutic purposes.
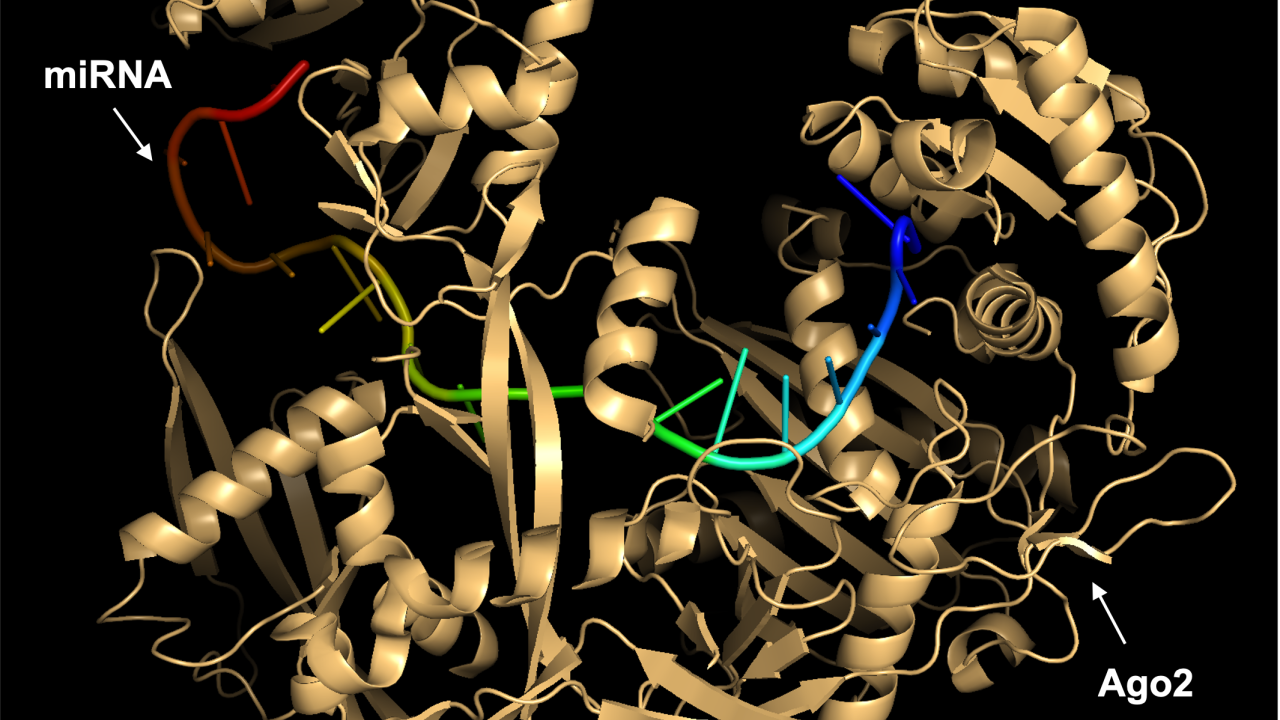
- RNA interference (RNAi). This approach utilizes a natural cellular process by which gene expression is silenced or reduced by the introduction of small RNA molecules such as small interfering RNAs (siRNAs) and microRNAs (miRNAs). After some initial processing via distinct molecular mechanisms, these molecules bind to a protein called Argonaute2 (Ago2) to form the RNA-induced silencing complex (RISC). They then bind to their complementary mRNA molecule, triggering a series of events that ultimately leads to mRNA degradation or prevention of mRNA translation into proteins.
- Antisense oligonucleotide (ASO). ASO-based therapeutics utilize short synthetic RNA molecules (antisense RNA) that bind to specific target RNA molecules. This binding can lead to a variety of consequences, including recruitment of cellular enzymes to degrade the target RNA (e.g. RNaseH1) or blocking the interaction of the target RNA with other molecules involved in its processing or translation.
- RNA aptamers. These are RNA molecules that can bind to specific target proteins with high affinity and specificity. They can be used as therapeutic agents to inhibit the activity of specific proteins.

RNA-based therapeutics offer several advantages over traditional small molecule drugs. RNA molecules are highly specific and can target individual genes or proteins, allowing for precise and targeted therapies. RNA-based therapeutics can also be synthesized in large quantities and can be rapidly developed, making them an attractive option for addressing new or emerging diseases as exemplified by the record-breaking development of mRNA vaccines for the COVID-19 pandemic. However, RNA-based therapeutics also face several challenges that must be addressed for them to become viable treatments. One of the most significant challenges is the delivery of RNA molecules to the target cells. As mentioned earlier, RNA molecules are typically unstable and can be rapidly degraded by nucleases, which can limit their efficacy. Additionally, RNA molecules are typically large and negatively charged, making them difficult to deliver to cells. RNA molecules can also potentially trigger immune responses in the body, leading to unwanted inflammation and side effects. Lipid nanoparticles (LNPs) and/or chemical modifications have been employed to tackle these issues and ongoing research efforts are focused on optimizing delivery methods, enhancing stability, and improving safety profiles of RNA-based therapeutics.

Despite the challenges, RNA-based therapeutics are showing promise in preclinical and clinical trials. Several RNA-based therapeutics have been approved by the FDA for the treatment of various diseases, including Alnylam’s RNAi-based Onpattro (Patisiran) and Givlaari (Givosiran) for the treatment of hereditary transthyretin amyloidosis and acute hepatic porphyria, respectively. Several other RNA-based therapeutics are currently in clinical trials for various diseases, including cancer, viral infections, and genetic disorders.
Apart from Alnylam, there are other biotech companies that are actively involved in the development and advancement of RNA-based therapeutics. A few notable examples are Moderna and BioNTech who developed the mRNA-based COVID-19 vaccines, Spikevax (Elasomeran) and Comirnaty (Tozinameran), respectively. Ionis Pharmaceuticals has also developed ASO-based drugs for diseases such as spinal muscular atrophy (SMA), hereditary angioedema (HAE), and amyotrophic lateral sclerosis (ALS). Many other biotechnology and pharmaceutical companies, both large and small, are investing in this rapidly evolving field to harness the potential of RNA for the development of innovative and targeted therapies.
In conclusion, RNA-based therapeutics represent a promising and rapidly advancing field in medicine. The unique properties of RNA, such as its ability to regulate gene expression, its versatility in targeting specific genes, and its potential for personalized medicine, have paved the way for the development of innovative treatments. RNA-based therapeutics offer advantages such as high specificity, potential for rapid development, and the ability to modulate previously undruggable targets. Despite the initial lag compared to DNA-based therapeutics, significant progress has been made in recent years, particularly with the success of mRNA-based vaccines against COVID-19. Ongoing research, technological advancements, and collaborations among academia, industry, and regulatory agencies will continue to propel the field forward. RNA-based therapeutics hold immense potential for treating a wide range of diseases, including genetic disorders, cancer, and infectious diseases, bringing us closer to a new era of precision medicine and improved patient outcomes.
References
Damase, T.R.; Sukhovershin, R.; Boada, C.; Taraballi, F.; Pettigrew, R.I.; Cooke, J.P. The limitless future of RNA therapeutics. Frontiers in Bioengineering and Biotechnology. 2021, 9:628137. https://doi.org/10.3389/fbioe.2021.628137
Kim, Y.K. RNA therapy: rich history, various applications and unlimited future prospects. Experimental and Molecular Medicine. 2022, 54:455-465. https://doi.org/10.1038/s12276-022-00757-5
Schirle, N.T.; Sheu-Gruttadauria, J.; McRae, I.J. Structural basis for microRNA targeting. Science. 2014, 346(6209):608–613. https://doi.org/10.1126/science.1258040
Zhu, Y.; Zhu, L.; Wang, X.; Jin, H. RNA-based therapeutics: an overview and prospectus. Cell Death and Disease. 2022, 13:644. https://doi.org/10.1038/s41419-022-05075-2
